From the R&D of a big international player an update on one of the most sensitive topics concerning the printing package: food-contact inks.
Authorities repeatedly impose strict regulations to ensure consumer safety in such sensitive applications as food contact materials and packaging. These serve a critical role in the food supply chain and there are rising concerns over the suitability and compliance of packaging inks, where the inks – and thereby the primary ink components like pigments, resins and additives – need to meet stringent requirements in terms of their toxicological profile and migration behavior. This article reviews organic pigments that are suitable for food packaging printing, in the light of the latest changes in legislation by the competent authorities, particularly the EU authorities.
Packaging Printing
Packaging has always served a critical role in the food supply chain and roughly 90% of all food is packaged, at least in mature countries. Due to the increasing amount of single packs and the needs for packaging in emerging markets the packaging sector is still growing, despite the tendencies to reduce packaging overall. Roughly 50% of globally consumed packaging is used for food, beverages, healthcare or personal care. All these fields of applications are considered sensitive and thus highly regulated. There is also a clear trend to more flexible packaging. According to a recent Pira study the global packaging market will amount to $841 billion sales in 2020 with flexible packaging growing at 2,6% CAGR to $182 billion sales. Besides sustainability and design, a major topic for food packaging relates to consumer health and food-safe packaging, including printing of food packaging. This is reflected in the increasing regulation of food packaging materials and the printing inks used to print on them, which is aiming to guarantee food safety and human health through use of appropriate packaging materials and printing technologies.
Food Contact Regulatory Landscape
Guidelines and regulations for food packaging differ from region to region and even from country to country, but the underlying principle is that the packaging should not endanger human health, change the composition of food or alter its organoleptic properties. Within the guidelines and regulations that concern food package printing inks for non-direct food contact (i.e. inks for use on the non-food contact surfaces of food packaging), there are few which are particularly important and enforced.
- For example, the EU Framework Regulation (EC) No. 1935/2004 states that food contact materials shall be safe. Also, the GMP Regulation (EC) No. 2023/2006, lays down the general rules for all business operators in the food packaging value chain, and specifies that quality assurance and control systems are established and implemented. The more recent EU plastics packaging regulation (EC) No. 10/2011 is relevant for food packaging printing inks as well, since printed plastics are also in scope. The plastic packaging market is growing, giving an additional importance to this regulation.
- A lot of attention is on Swiss Ordinance SR 817.023.21, which was recently updated in May 2017 changing Annex 6 to Annex 10. Annex 10 now comprises both List A and List B of the former Annex 6. All A-substances have been thoroughly evaluated in terms of their health and safety risk and have a stated Specific Migration Limit (SML) or need to comply with the Overall Migration Limit (OML) of 60 ppm. B-substances for which there is insufficient information currently to define a SML can be used provided they comply with a migration threshold of maximum 10 ppb.
- EuPIA (European Printing Ink Association) provides general guidelines, including directions on Good Manufacturing Practice, and a negative list of banned ink chemicals. Worth mentioning is the fact that the EU commission started activities to adopt a new Union legislation on printed food contact materials, including printing inks and commonly printed materials such as paper and board, in 2018. These activities followed the initially German approach of a German Ink Ordinance aimed not only at printing inks for non-direct food contact, but also direct and transient direct food contact.
- In the US, the FDA requires no pre-market clearance provided no migration is taking place. The food contact notification (FCN) process in place covers plastic mass-coloration and coatings, but not printing inks. For printing inks read-across from coatings is accepted.
- In Japan, under the Food Sanitation Law, the inertness of food packaging must be ensured and the Japanese Printing Manufacturers Association has issued recommendations on food packaging. According to the latest information Japan is moving toward adopting a legal positive list system for food contact materials.
- In China, the Food Safety Law with its Food Safety Standards (positive lists) has been updated in 2016. A food safety standard for printed materials is currently being set up to be included.
- Also, some brand owners (e.g. Nestlé) have defined their own guidelines for food packaging printing, which must be complied with by their packaging suppliers.
As far as pigments are concerned, the primary concern of regulations regarding food packaging is to define upper limits for impurities such as traces of heavy metals, primary aromatic amines (PAAs), polychlorobiphenyls (PCBs) or dioxins. In Europe, the most relevant recommendations concerning colorants for food packaging are the European Resolution AP (89)1 and the German BfR IX.
Ink Migration
A major concern with printed food packaging, when it comes to food safety and consumer health, is ink migration. All printing inks have the potential to migrate from the printed side of the packaging to the food-contact side and contaminate the food. At this point, it must be emphasized that ink migration into the packaged food is not only a function of the ink itself, but also largely depends on the other packaging components (print substrate), the printing and processing conditions, and on the nature of the packaged food (e.g., fatty foodstuffs are more prone to absorb ink migrants). In the end, it is the entire printed packaging together with its content that must be compliant with regulations and to ensure consumer safety, not just the ink. Ultimately, the responsibility lies with the food packer.
 Generally, it is always recommended to use barrier substrates such as glass or metal or implement functional barrier layers (aluminum laminated film or cardboard, for example), even though this doesn’t resolve the set-off migration issue. Also, low-migratory ink components should be selected whenever possible, and if migrating substances cannot be avoided, comprehensive toxicological data must be available and migration levels must be below the defined SML.
Generally, it is always recommended to use barrier substrates such as glass or metal or implement functional barrier layers (aluminum laminated film or cardboard, for example), even though this doesn’t resolve the set-off migration issue. Also, low-migratory ink components should be selected whenever possible, and if migrating substances cannot be avoided, comprehensive toxicological data must be available and migration levels must be below the defined SML.
Ink migration and thereby food contamination can occur in several ways. The most common migration mechanisms are set-off, diffusion and gas phase migration. Set-off might occur when the prints are stacked or rolled up after printing and before conversion. In that case, ink components might transfer directly from the printed side to the reverse side of the packaging and later be in direct contact with food. In case of diffusion migration, ink compounds might reach the food by diffusing through the packaging substrate (e.g. thin-walled PE pouches). Gas phase migration might occur during strong heating (e.g. microwave-ready meals).
Examples of ink components with high migration potential include low molecular weight photoinitiators and monomers, as present in UV-curable inks, solvents, plasticizers, as well as any other low molecular weight substances (< 1’000 g.mol-1). In general, ink components like binders, inorganic materials (e.g. fillers, silica matting agents) and organic pigments have no or extremely low migration potential and are usually not regarded as migrants. That said, aforementioned non-migrants might contain impurities which could migrate and contaminate food. In case of organic pigments, such impurities often consist of substances with significant toxicity and human health risks such as Primary Aromatic Amines (PAAs) or Polychlorinated Biphenyls (PCBs).
Azo Pigments and PAAs
Azo pigments still reign supreme amongst organic pigments and represent more than 50% of the worldwide organic pigment production by volume. They are used in all kind of industries and applications. In printing inks, the process colors and spot colors or Pantone® shades have traditionally been based on azo pigments.
All azo pigments can potentially contain traces of PAAs, which are relatively small, mostly hydrophobic aromatic molecules that are prone to migrate, unlike the pigment itself (due to its particulate, crystalline nature). This is related to the azo pigment manufacturing process, which always starts with the conversion of a PAA into a diazonium salt in the first synthesis step (the so-called diazotization). In addition, the coupling component introduced in the second step -– while not being a PAA itself -– might also contain PAA residues.
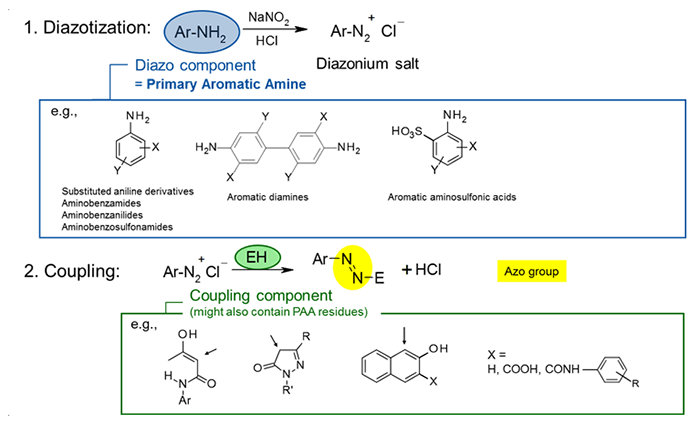
Considering possible PAA residues originating from the coupling component and unreacted PAA from the diazotization step, the final azo pigment might contain residual PAAs at its surface or embedded into the crystal lattice (even after thorough washing). On top of that, certain non-azo pigments are also made from PAAs and can also potentially include PAA impurities. All in all, there are numerous pigments, mostly azos but some non-azo types as well, that are potentially contaminated by PAA residues, especially yellow, orange, red and magenta pigments (cyan and green pigments of the copper-phthalocyanine type or DPP (Diketo-pyrrolo-pyrrole) red pigments, for example, are not concerned).
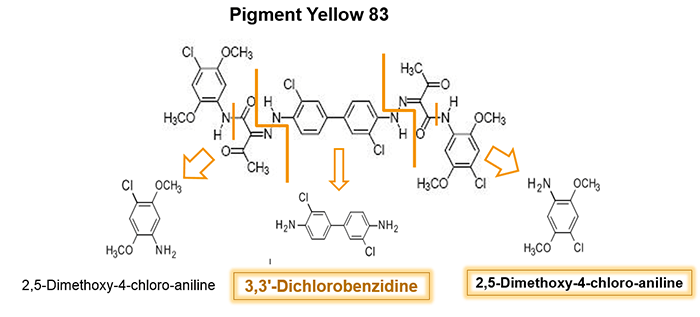
Most of PAAs exhibit acute or chronic toxicity, and several are suspected human carcinogens. At least 22 PAAs are recognized and classified as CMR (Carcinogenic, Mutagenic, Reprotoxic) substances and are not allowed to come into contact with food at all. Examples of CMR-classified PAAs include 3-3’-Dichlorobenzidine (used in the synthesis of PY 13, PY 83 and other diarylide pigments) and o-Anisidine (used in the synthesis of e.g. PY 74). Both PY 83 and PY 74 are very popular yellow ink pigments.
PAA Regulations in Printed Food Packaging Materials
Older regulations and recommendations about PAAs are usually dealing with PAA content of the pigment itself (e.g. AP (89) 1 or EuPIA 2011), whereas for more recent regulations like BfR IX (2015) or (EC) No. 10/2011, it is the final packaging item that is in scope and it is PAA migration to food which is defined and regulated. For instance, under European Resolution AP (89) 1 (1989), the maximum threshold limit for PAAs in pigments is 500 ppm in total and 10 ppm for 3 specific PAAs (singly and in total). And, in the German BfR IX recommendation from October 2015, limit values for PAAs are no longer defined for pigments but for migration from final article, with a migration limit of 10 ppb in total and only 2 ppb singly for 22 specific PAAs that are recognized as CMR substances. According to BASF Colors & Effects’ worst-case assessment, aforementioned migration limits for PAAs translate into PAA content requirements for pigments which are significantly lower compared to the 500 ppm limit as recommended under AP (89) 1 or the old BfR IX recommendation from 2010. Thus, maximum PAA content of the pigment should now be less than 100 ppm singly and in total and 20 ppm singly for 22 PAAs classified as CMR.
New Analytical Method for PAA Analysis in Pigments
Following the recent change in BfR IX recommendation regarding PAAs, and considering similar European regulations which are also focusing on PAA migration, there was an urgent need for a new analytical method to accurately identify and quantify PAAs as single substances in organic pigments. The established method from ETAD (Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers), ETAD No. 212, became obsolete since it did not anymore fulfil the criteria regarding sample preparation, extraction, selectivity and detection limit. Since no industry-wide feasible and accepted analytical method existed that complied with the newly required benchmarks, during 2015 and 2016, BASF developed a new test method for the exact qualitative and quantitative analysis of PAAs in pigments. BASF promoted the test method at ETAD, who had it validated by a round robin test within ETAD members. The method was subsequently published as ETAD Method No. 212 revised, edition May 3rd, 2016. As a released ETAD method, industry-wide acceptance is ensured and conversion into a norm is intended. All ETAD members, which include all major pigment and dye manufacturers, have the right to use the method free of charge, while non-ETAD members can purchase the method.
Conclusion
Increasing and stricter regulations for food contact materials around the globe and the focus on migration of impurities increase the necessity of compliant, high purity pigments for printing inks. BASF Colors & Effects has installed a spot testing regime which covers the regulated impurities and checks all products in its portfolio for food contact applications accordingly. Additional measures are raw material specifications and a management of change process ensuring compliance. For all products supported for food contact applications in printing inks, detailed food contact statements are available and regularly updated. BASF Colors & Effects dedicated team of technical experts and product stewards facilitates compliance along the entire value chain of printed food packaging.
di Ruth Bauer, BASF Colors & Effects GmbH, Ludwigshafen, Germany, e Stephane Biry, BASF Colors & Effects Switzerland AG, Basel, Switzerland

















